Main scientific interests of the Hoch group:
Combinations of ionic and metallic bonding within crystalline solids
|
| Our different working fields have one thing in common -- we create unusual bonding situations. Our interest lies in the creation of mixed chemical bonds with metallic and ionic contributions. Materials with unusual structural features have unusual physical properties. Some of the systems we care about are 'bad metals', which means, they are indeed metals, but the specific resisitivity is several orders of magnitude higher than the one of 'normal' metals, and their resistivity does not increase linearly with temperature. Other compounds of interest show very good metallic behaviour, but their crystal structures clearly show compartments with ionic building blocks alternating with compartments with metallic bonds. |
 |
 |
Amalgams of electropositive metals: model systems for 'bad metallic behaviour' in polar intermetallic phases When combining electropositive metals (e.g. alkaline, alkaline earth or lanthanoid metals) with mercury, amalgams form. The formation of Zintl-like compounds with high ionic character could be expected due to the high electronegativity difference. However, the electron transfer is in all cases considerable but never complete. This is impeded by the low electron affinity of mercury. The resulting amalgams are metallic, but show the very special behaviour of 'bad metals', common to highly polar intermetallic phases. The combination of metallic and ionic contributions is important for materials with special features due to unconventional thermoelectric, magnetic, optic, catalytic and electric properties. |
| Subvalent compounds: spacial separation of ionic and metallic building blocks Reactions of cesium suboxides with metal oxides can result in the formation of suboxometalates. In analogy, reactions of barium subnitride with metal nitrides can yield subnitridometalates. In both families of compounds, the crystal structures contain metalate anions which look exactly like those in normal metalates (silicates, aluminates, ferrates, you name it), and also metallic building units which look like cut-outs of metal structures or intermetallic phases. Both structural building units combine to common crystal structures and show overall good metallic behaviour. The trick is now to deliberately tune the dimensions of the ionic and the metallic parts in order to create anisotropic behaviour. |
 |
 |
In order to prepare the special chemical compounds we are interested in, we have to apply special preparative methods.Working with mercury, cesium, barium and all the very reactive compounds or elements affords for glove box or Schlenk techniques. Many of our compounds are not only highly sensitive towards air and moisture, but also do not like high temperatures. Our reactions normally take place at - for a solid state chemist - low temperatures. We have developed special electrochemical preparation techniques and low-temperature reactions, and further development of preparative ways towards new compounds are part of the daily work. |
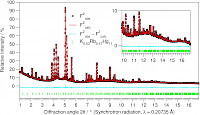
| We characterise our new compounds structurally by X-ray techniques: single crystal and powder diffraction are basic routine, reaction control and studies on phase changes and stability ranges are performed via DSC/DTA/TG and temperature-dependent powder diffraction. Physical properties of interest are electric conductivity, magnetic susceptibility, and also Mössbauer spectroscopy in suitable cases. In addition, vibrational spectroscopy, NMR spectroscopy and other more specialised analyses are of great help to get a more and more precise picture of the structure-property relations for our compounds. In order to get insight in the electronic structures we perform band structure calculations. |
 |
 Group of Dr. Constantin Hoch - Faculty for Chemistry and Pharmacy
Group of Dr. Constantin Hoch - Faculty for Chemistry and Pharmacy
 Group of Dr. Constantin Hoch - Faculty for Chemistry and Pharmacy
Group of Dr. Constantin Hoch - Faculty for Chemistry and Pharmacy




